What is FMEA?
Failure Modes and Effects Analysis (FMEA) is a process used to identify possible failures in a design, process, product, or service. It is used as a tool to document and guide design decisions for new products and processes and when changes are made to or effect those products and processes. You may also hear FMEA used interchangeably with FMECA. FMECA adds a criticality analysis step to identify possible failures that may be mission critical.
- Failure modes are the ways in which something may fail.
- Effects analysis is the analysis of the failure and it’s effects.
FMEA was initially developed by the military. It was later used on many NASA programs and within other governmental organizations such as USGS and EPA. FMEA is also commonly seen in the automotive, healthcare, software, and materials science industries.
Why Use It?
Failure in healthcare processes and products has the potential to result in loss of human life. Whether you’re designing the software that will run a portable defibrillator or updating your quality reporting processes to meet new regulations, FMEA can help to ensure all possible failure modes are identified and eliminated or minimized as soon as possible. It can also help design the system so it has less overall likelihood of failure, ensure failure is contained to an acceptable level, and assist the design and development teaming making design decisions.
Pros:
- Improve quality
- Increase customer satisfaction
- Reduce repeated failures
- Better understand the process/product/design/service
Cons
- Time consuming and complex to complete
- Harder to use when multiple departments/organizations are involved
- Harder to use if multiple failures occur in one subsystem
When Use It?
FMEA/FMECA should be first used when a new design, process, product, or service is beginning to be planned and designed. It should also be dusted off when changes are made to a design, process, product, or service – such as when organizational changes affect processes, new rules and regulations come in to play, or when the product is updated, among others.
How to use it?
*The below steps are generalized for completing a FMEA on a process and are taken from https://rsdo.gsfc.nasa.gov/documents/rapid-iii-documents/mar-reference/gsfc-fap-322-208-fmea-draft.pdf. For more FMEA analysis methods, see the aforementioned link.
Let’s Get Started…
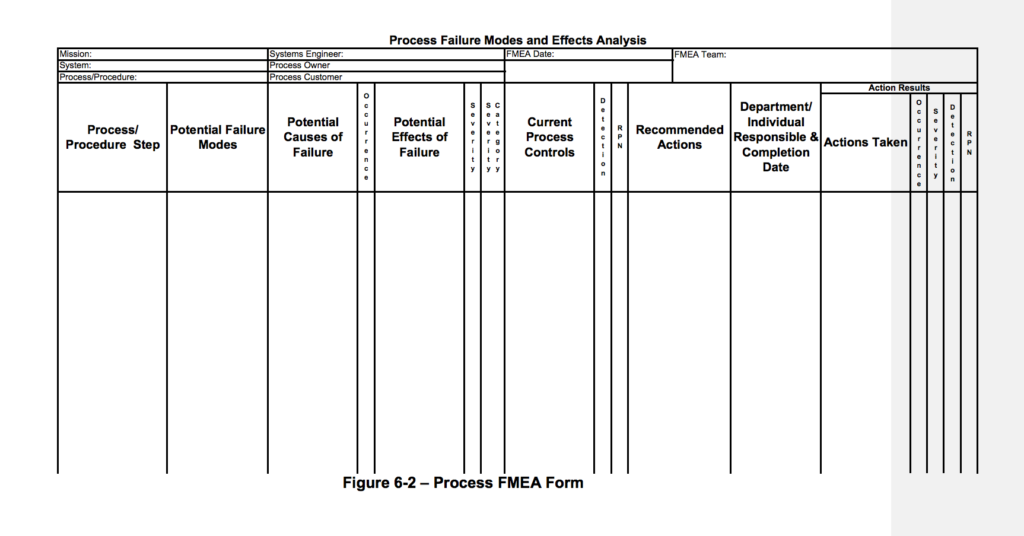
1.) Gather process information and fill in in the document header
2.) Identify the name of the process or procedure and record it in the first column.
3.) Identify all possible modes of failure the identified process may incur. Write these in the second column.
4.) For each possible failure mode, list the potential causes of failure in the third column. These should be specific and described in terms of something that can be controlled or corrected.
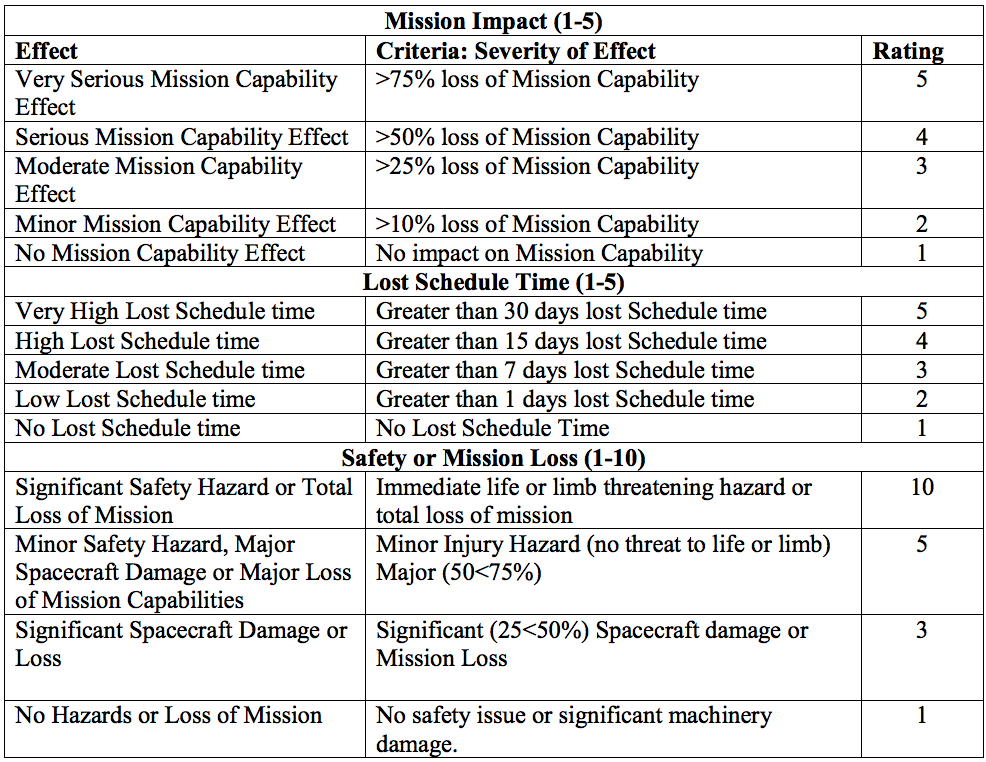
- Assign each potential cause of failure an occurrence ranking in the “occurrence” column.
- Below is an example occurrence probability chart
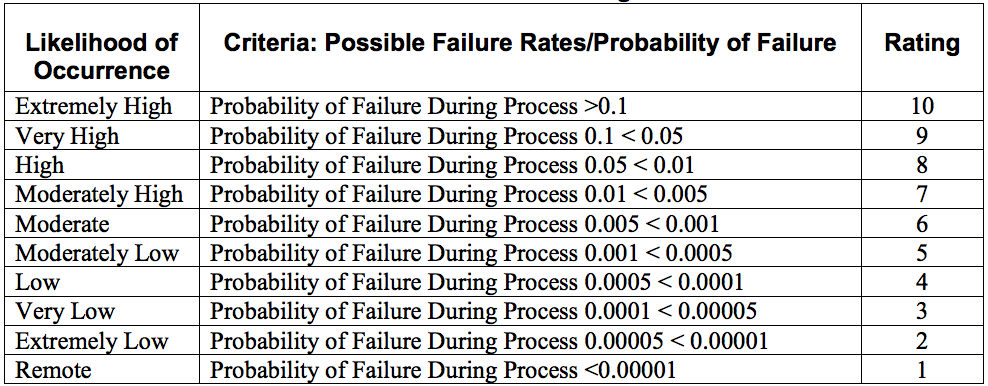
5.) List the effects of what would happen if the failure did occur ((i.e. # of hours rework or extra tasks, patient safety issues, fines)
- Assign each potential effect of failure a severity ranking. (Note: these are from NASA, so while it’s unlikely your healthcare process will result in spacecraft damage…you get the point)
6.) If the severity is rated 9 or 10, enter the letters “OS” in column 7 of the Process FMEA form. This potential failure mode must be addressed for the safety of the process.
7.) In the current process controls column, identify any mechanisms that are in place to detect, prevent, or minimize the impact of the failure.
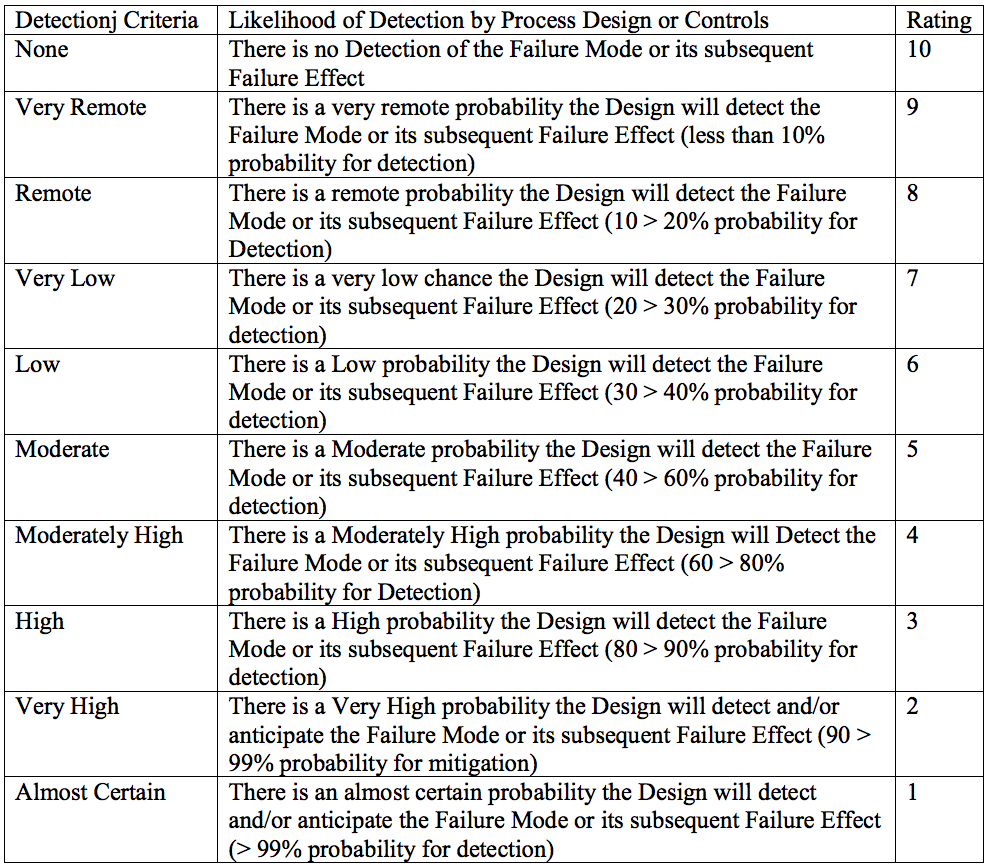
8.) Assign the failure mode a detection ranking based on the criteria below:
9.) Calculate an RPN number by multiplying the occurrence, severity, and detection rankings. Failure modes with the highest RPNs should be addressed first.
10.) In the recommended actions column, list ways to reduce the occurrence, severity, and/or prevention rankings for the failure mode so that the RPN number may be reduced. If there is no action required, still write – “no action required”. Do not leave this column blank.
11.) Assign a responsible party. As mentioned before, items with the highest RPNs should be addressed first.
12.) Once action has been taken, update the remaining columns.
All done! Well, until you have to update it…
Other things to think about when implementing a FMEA include…
- Will the failure be caught?
- How long may a failure go unnoticed?
- Will it be apparent when something fails?
Practice Question Time
A hospital group performs a FMEA analysis on its inpatient admission process. It finds two main failure modes; one where patients do not fully complete health history information and are incorrectly placed, and another where patients status were improperly designated (i.e observation vs inpatient) at admission. Each failure mode was assigned the following occurrence, severity, and detection rankings:
|
Occurrence Ranking
|
Severity Ranking
|
Detection Ranking
|
|
|
Incorrect Placement
|
6
|
6
|
5
|
|
Incorrect Status
|
4
|
7
|
6
|
Based on this FMEA analysis, which failure mode should be addressed first?
A.) Incorrect placement, because it occurs more frequently and is harder to detect.
B.) Incorrect status, because its severity is higher
C.) Incorrect placement, because its RPN number is highest
D.) Incorrect status, because its RPN number is lowest
Answer:
Because we’re doing a FMEA analysis, we want to first address failure modes with the higher RPNs. As mentioned above, to find the RPN number we multiply the occurrence ranking, severity ranking, and detection ranking. In this scenario we find that incorrect placement has a RPN of 180 while incorrect status has a RPN of 168. Since incorrectly placing a patient has a larger RPN in this scenario than incorrectly indicating status, answer C is our best choice.
You can find more practice questions like this here.
Additional Resources
NASA Preferred Reliability Practices – Failure Modes, Effects and Criticality Analysis
ASQ Overview of FMEA